Apocynaceae
Description
Large to small trees, shrubs, small to very large woody climbers, perennial or, rarely, annual herbs.
Leaves simple, mostly entire, rarely crenulate or with spines at the margin (but not in Malesia);
— In Malesia the leaves of Apocynaceae, subfamily Rauvolfioideae, may be spirally arranged, in whorls or, most commonly, opposite. In all other subfamilies they are primarily opposite, and only very rarely in whorls (in Malesia only in Parsonsia and possibly, rarely, in Parameria). Stipules, strictly speaking, are absent but there is often a raised interpetiolar line and in some Alstonia species and in Dyera there are small intrapetiolar stipule-like structures. In Chilocarpus, Tabernaemontana, and Voacanga there are small intrapetiolar ocrea. Colleters, which are small awl-shaped glands, are very often present in the axils of the petioles with the leaves and sometimes in a ring around the node on the interpetiolar line. In some genera these glands may also be found on the petiole and the leaf blade but not in Malesian Rauvolfioideae and Apocynoideae. It is unclear if there are any species that are deciduous in Malesia although some species of Wrightia, particularly W. pubescens subsp. lanitii, are known to be deciduous further north in seasonal climates and may be so in the more seasonal parts of Malesia such as the Lesser Sunda Islands. The leaves are always simple and, in Malesia, almost always entire (but see Dyera). The leaves of most species are coriaceous to varying degrees although thinner leaves are known in many genera. Leaves of remarkably different shapes are known within species in Chilocarpus, Micrechites and Parsonsia (and possibly in Willughbeia) and may simply be unrecorded in other genera. Changes in leaf shape may be due to differing levels of maturity, growth form (for species that may scramble or climb depending on conditions) or response to light. A number of species from several genera have leaves which are punctate beneath. This is a character for all species in Chilocarpus and Leuconotis but is a useful diagnostic character for species within other genera such as Anodendron and Alyxia. The venation is always pinnate and in most species there is an intramarginal vein although this is rarely strong enough to be a noticeable feature.
Inflorescence cymose, rarely fasciculate or flowers solitary;
Flowers hermaphrodite;
Sepals often with colleters inside.
Stamens inserted on the inside of the corolla tube;
Fruit a drupe, berry, capsule or follicle.
— The seeds of all species of subfamily Apocynoideae are comose with the exception of Eucorymbia which lacks a coma. The coma is most commonly micropylar, i.e. with the coma directed towards the apex of the follicles. In Kibatalia and Wrightia the coma is chalazal, i.e. directed towards the base of the fruit. In Strophanthus there is a coma at both ends although the chalazal coma is generally smaller and easily falls off. The grain of most species is somewhat flattened and more often glabrous than pubescent. The seeds of Rauvolfioideae are much more variable and may be simple, ciliate, winged or arillate. Seeds in both subfamilies provide useful taxonomic characters.
Seeds simple, arillate, winged, with a ciliate margin or with an apical and/or basal coma.
Seeds simple, arillate, winged, with a ciliate margin or with an apical and/or basal coma.
Distribution
The Apocynaceae s.l. is one of the ten largest angiosperm families with about 4000 species in about 425 genera. It is found throughout the world although very much more diverse in the tropics. This work deals only with subfamilies Rauvolfioideae (c. 915 species in 84 genera) and Apocynoideae (c. 822 species in 77 genera), both of which have the same distribution as the family as a whole.
The largest genera within these two subfamilies are Mandevilla (c. 150 species) in the Americas, Alyxia (c. 106 species) in Southeast Asia, Australia and the islands of the Pacific, Tabernaemontana (c. 100 species), which is pantropical, and Aspidosperma (c. 70 species) in the Americas.
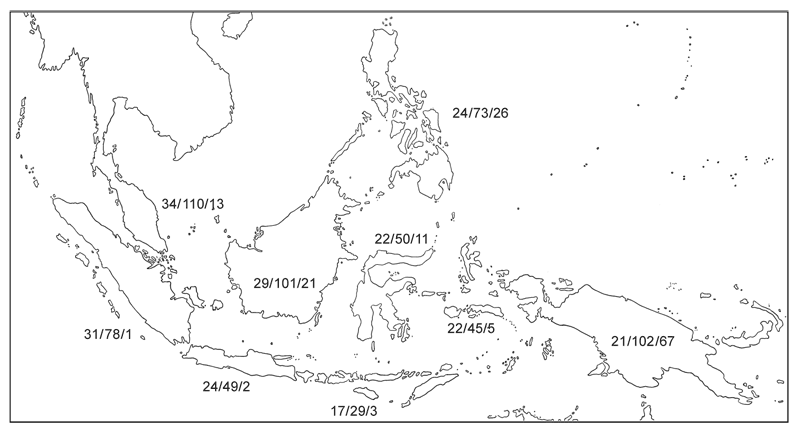
In Malesia there are 43 native genera in these two subfamilies. The largest genus in Malesia is Alyxia with 55 species followed by Parsonsia with 27 species (one with 7 varieties), Kopsia with 18 species and 2 varieties, Alstonia with 16 species, Kibatalia with 15 species, and Anodendron and Tabernaemontana with 14 species each. Altogether there are 295 native species in Malesia, comprising 304 taxa when one includes infraspecific taxa. There is a decrease in the number of genera from West to East with 34 genera in Peninsular Malaysia and only 21 genera in New Guinea. Species and infraspecific taxon diversity is highest in Peninsular Malaysia with 109 taxa and New Guinea with 108 taxa. Endemism is particularly high in the Philippines and New Guinea. Diversity and endemism is surprisingly low in Sumatra but this may be an artefact of collecting and with better collecting the known diversity may go up (with a possible corresponding decrease in endemism in Peninsular Malaysia if some of those species turn up in Sumatra).
The largest genera within these two subfamilies are Mandevilla (c. 150 species) in the Americas, Alyxia (c. 106 species) in Southeast Asia, Australia and the islands of the Pacific, Tabernaemontana (c. 100 species), which is pantropical, and Aspidosperma (c. 70 species) in the Americas.
In Malesia there are 43 native genera in these two subfamilies. The largest genus in Malesia is Alyxia with 55 species followed by Parsonsia with 27 species (one with 7 varieties), Kopsia with 18 species and 2 varieties, Alstonia with 16 species, Kibatalia with 15 species, and Anodendron and Tabernaemontana with 14 species each. Altogether there are 295 native species in Malesia, comprising 304 taxa when one includes infraspecific taxa. There is a decrease in the number of genera from West to East with 34 genera in Peninsular Malaysia and only 21 genera in New Guinea. Species and infraspecific taxon diversity is highest in Peninsular Malaysia with 109 taxa and New Guinea with 108 taxa. Endemism is particularly high in the Philippines and New Guinea. Diversity and endemism is surprisingly low in Sumatra but this may be an artefact of collecting and with better collecting the known diversity may go up (with a possible corresponding decrease in endemism in Peninsular Malaysia if some of those species turn up in Sumatra).
 |
Pollination
The pollination biology of species of Apocynaceae, subfamilies Rauvolfioideae and Apocynoideae, in Malesia has not been reported in any detail. In the family as a whole detailed studies are rare. Most detailed are in the genera Asclepias (see Wyatt & Broyles 1997), Rauvolfia (Koch et al. 2002) and Mandevilla (Torres & Galetto 1998). Albers & Van der Maesen (1994) have reported more generally on the pollination biology of some West African native and cultivated species.
The flowers of Apocynaceae are bisexual and protandrous. In a few cases (in Rauvolfia and Carissa) the flowers are reported to be functionally dioecious (Koch et al. 2002). This condition could be much more widespread in the family than has been appreciated. Boiteau & Allorge (1978) suggested that the species they placed in their subfamily Plumerioideae (= Rauvolfioideae but with a narrower circumscription) were autogamous and that the species they placed in subfamily Tabernaemontanoideae (= Rauvolfidoideae, tribe Tabernaemontaneae) were cross pollinators. Subsequent work has not supported this difference although self-compatibility has been reported in a few species (Albers & Van der Maesen 1994; Koch et al. 2002).
The Apocynaceae s.l. contains a wide range of floral morphologies reflecting a diversity of potential pollination systems. These range from the relatively simple in subfamily Rauvolfioideae to the extremely complex pollination systems in species of subfamily Asclepiadoideae. The basic trend is for more complex floral morphologies and increased synorganisation within the androecium, and between the androecium and the gynoecium, from the Rauvolfioideae to Apocynoideae to Periplocoideae to Secamonoideae to Asclepiadoideae. However, the phylogenetic relationships between these subfamilies is not entirely clear resulting in a lack of detailed knowledge of the evolutionary relationships between taxa with these morphologies and the pollination pressures which may have led to their evolution.
In the Rauvolfioideae and Apocynoideae the pollen is shed shortly before anthesis and may be secondarily presented on the style head. Albers & Van der Maesen (1994) have demonstrated that the tip of the style head is always unreceptive to pollen in Rauvolfioideae and Apocynoideae. In the Rauvolfioideae, where the stamens are not attached to the style head, the corolla may fall off shortly after the male phase (which often leads to large collections of herbarium specimens with almost no open corollas for examination!). In the Apocynoideae, where the stamens are attached to the style head, the corolla is generally longer lasting.
In the Apocynoideae the margins of the anthers are lignified and the anthers are adnate to the style head. There is only a narrow slit between one anther and the next and the lignified margins likely act as guide-rails for pollinators. As the anthers are also adnate to the style head, effectively forming a plug in or at the top of the corolla tube, the 5 slits between the anthers are the only way to reach the nectar at the base of the flower. In the Rauvolfioideae the morphology is not as complex but with the position of the anthers and sometimes with internal hairs, and in a few cases with corona lobes, a similar function is achieved. In some genera the corolla is not salverform and the pollination system not so readily apparent. In Beaumontia the anthers remain adnate to the style head but the corolla is so large and open and the filaments and style are so long that there is no barrier for any pollinator to enter the flower. How pollination is effected is unclear but may be by flies.
Albers & Van der Maesen (1994) suggest that more or less all Apocynaceae, subfamilies Rauvolfioideae and Apocynoideae, are pollinated by Lepidopteran or Hymenopteran insects. They point to their own field studies and to the long narrow tubes of most species as evidence for this. It would certainly take an extremely long-tongued animal to reach the nectar of species such as Kopsia flavida with a corolla tube up to 4.9 cm long. Pollen transfer is likely to be on the mouth parts.
Fruit dispersal in Malesian Apocynaceae has largely to be inferred from the fruit morphology rather than because we have much direct evidence, especially in subfamily Rauvolfioideae. All species in subfamily Apocynoideae (with the exception of Eucorymbia), plus Alstonia and Dyera from subfamily Rauvolfioideae, have dehiscent fruits and wind blown seeds. In the Apocynoideae the seeds have a coma of hairs at one or both ends, in Alstonia the seeds have a ciliate margin, and in Dyera they have a wing, all morphological adaptations to wind dispersal. I can attest to how effective this can be when on many occasions having found seeds on the ground I have subsequently been unable to find the parent plant, suggesting the plant might be quite far away. The fruit of Cerbera and Ochrosia species are mostly very fibrous or have air-filled cavities, possibly adaptations to dispersal by water. Many species in both genera are riverine or littoral in their distribution which would reflect this. Most species in subfamily Rauvolfioideae, however, are probably dispersed by birds or mammals. Most of them have fleshy berries or drupes and some also a fleshy aril around the seed. There remain, though, many unknowns to explain fruit and seed morphology in the Apocynaceae. It is unclear what would be able to penetrate the hard fruit wall of species of Melodinus to access the pulp around the seeds within but may be large-billed birds or rodents. Chilocarpus fruits develop as fleshy berry-like fruit suggesting animal dispersal but ultimately they dehisce to expose seeds that have a corky aril. What then would be attracted to these arillate seeds is not known. Why do the seeds of Eucorymbia alba from subfamily Apocynoideae not have a coma and without a coma how are the seeds dispersed from the dehiscent fruits?
The flowers of Apocynaceae are bisexual and protandrous. In a few cases (in Rauvolfia and Carissa) the flowers are reported to be functionally dioecious (Koch et al. 2002). This condition could be much more widespread in the family than has been appreciated. Boiteau & Allorge (1978) suggested that the species they placed in their subfamily Plumerioideae (= Rauvolfioideae but with a narrower circumscription) were autogamous and that the species they placed in subfamily Tabernaemontanoideae (= Rauvolfidoideae, tribe Tabernaemontaneae) were cross pollinators. Subsequent work has not supported this difference although self-compatibility has been reported in a few species (Albers & Van der Maesen 1994; Koch et al. 2002).
The Apocynaceae s.l. contains a wide range of floral morphologies reflecting a diversity of potential pollination systems. These range from the relatively simple in subfamily Rauvolfioideae to the extremely complex pollination systems in species of subfamily Asclepiadoideae. The basic trend is for more complex floral morphologies and increased synorganisation within the androecium, and between the androecium and the gynoecium, from the Rauvolfioideae to Apocynoideae to Periplocoideae to Secamonoideae to Asclepiadoideae. However, the phylogenetic relationships between these subfamilies is not entirely clear resulting in a lack of detailed knowledge of the evolutionary relationships between taxa with these morphologies and the pollination pressures which may have led to their evolution.
In the Rauvolfioideae and Apocynoideae the pollen is shed shortly before anthesis and may be secondarily presented on the style head. Albers & Van der Maesen (1994) have demonstrated that the tip of the style head is always unreceptive to pollen in Rauvolfioideae and Apocynoideae. In the Rauvolfioideae, where the stamens are not attached to the style head, the corolla may fall off shortly after the male phase (which often leads to large collections of herbarium specimens with almost no open corollas for examination!). In the Apocynoideae, where the stamens are attached to the style head, the corolla is generally longer lasting.
In the Apocynoideae the margins of the anthers are lignified and the anthers are adnate to the style head. There is only a narrow slit between one anther and the next and the lignified margins likely act as guide-rails for pollinators. As the anthers are also adnate to the style head, effectively forming a plug in or at the top of the corolla tube, the 5 slits between the anthers are the only way to reach the nectar at the base of the flower. In the Rauvolfioideae the morphology is not as complex but with the position of the anthers and sometimes with internal hairs, and in a few cases with corona lobes, a similar function is achieved. In some genera the corolla is not salverform and the pollination system not so readily apparent. In Beaumontia the anthers remain adnate to the style head but the corolla is so large and open and the filaments and style are so long that there is no barrier for any pollinator to enter the flower. How pollination is effected is unclear but may be by flies.
Albers & Van der Maesen (1994) suggest that more or less all Apocynaceae, subfamilies Rauvolfioideae and Apocynoideae, are pollinated by Lepidopteran or Hymenopteran insects. They point to their own field studies and to the long narrow tubes of most species as evidence for this. It would certainly take an extremely long-tongued animal to reach the nectar of species such as Kopsia flavida with a corolla tube up to 4.9 cm long. Pollen transfer is likely to be on the mouth parts.
Fruit dispersal in Malesian Apocynaceae has largely to be inferred from the fruit morphology rather than because we have much direct evidence, especially in subfamily Rauvolfioideae. All species in subfamily Apocynoideae (with the exception of Eucorymbia), plus Alstonia and Dyera from subfamily Rauvolfioideae, have dehiscent fruits and wind blown seeds. In the Apocynoideae the seeds have a coma of hairs at one or both ends, in Alstonia the seeds have a ciliate margin, and in Dyera they have a wing, all morphological adaptations to wind dispersal. I can attest to how effective this can be when on many occasions having found seeds on the ground I have subsequently been unable to find the parent plant, suggesting the plant might be quite far away. The fruit of Cerbera and Ochrosia species are mostly very fibrous or have air-filled cavities, possibly adaptations to dispersal by water. Many species in both genera are riverine or littoral in their distribution which would reflect this. Most species in subfamily Rauvolfioideae, however, are probably dispersed by birds or mammals. Most of them have fleshy berries or drupes and some also a fleshy aril around the seed. There remain, though, many unknowns to explain fruit and seed morphology in the Apocynaceae. It is unclear what would be able to penetrate the hard fruit wall of species of Melodinus to access the pulp around the seeds within but may be large-billed birds or rodents. Chilocarpus fruits develop as fleshy berry-like fruit suggesting animal dispersal but ultimately they dehisce to expose seeds that have a corky aril. What then would be attracted to these arillate seeds is not known. Why do the seeds of Eucorymbia alba from subfamily Apocynoideae not have a coma and without a coma how are the seeds dispersed from the dehiscent fruits?
Taxonomy
Attempts to maintain consistency in species concept across the family have been made. However, with different numbers and quality of specimens to contend with, markedly different suites of characters to evaluate, different taxonomic histories for the taxa studied to take into account, and my accumulated understanding of characters and biogeographic patterns over the period of time that this work was undertaken, to state that all taxa have been evaluated on the same set of criteria would be foolish. I would hope though that my use of the rank of species is fairly consistent even if I could not guarantee it. My use of the rank of subspecies is when I believe a single species can be separated into one or more entities on at least one observable morphological difference and there is no geographical overlap between them. A variety has the same degree of morphological distinctness but is not geographically distinct to the same degree. Again there is a large degree of subjectivity in this assessment coupled, in this region, with the fact that large areas are inadequately collected and it is, therefore, not always possible to tell whether distribution patterns of taxa are real or an artefact of where collecting has occurred. For example in Parsonsia sanguinea a number of varieties are recognised, some of which show distinct geographical isolation suggesting that they may better be recognised as subspecies. However, the highlands of New Guinea, where this species occurs, are known to be inadequately collected so the geographical isolation of these taxa and, indeed, the morphological basis for the taxa may disappear as more and better collections are made. Lastly I might add that I have on the whole been rather reluctant to use infraspecific taxa at all, precisely for the reason that it seems to assume a level of understanding of patterns of variation and distribution that are simply not possible with the herbarium material currently available.
Subfamilies Rauvolfioideae and Apocynoideae form the Apocynaceae as traditionally delimited. A key to all the subfamilies, including those traditionally placed in the Asclepiadaceae, Periplocoideae, Secamonoideae and Asclepiadoideae, is given later along with a further discussion on the relationships within the family.
The Apocynaceae is in the Gentianales along with the Rubiaceae, Gentianaceae, Loganiaceae and Gelsemiaceae (see Backlund et al. 2000). Within the order it is characterised by the possession of latex. The latex is most often white, less often clear, yellowish or bluish, and only rarely absent.
The Apocynaceae is a family in flux due to recent advances made in our understanding of the relationships between and within the families of the Gentianales through molecular phylogenetic studies (Sennblad & Bremer 1996, 2000, 2002; Endress et al. 1996; Sennblad et al. 1998; Potgieter & Albert 2001; Livshultz et al. in press; Simões et al. in press). The principal effect of this is the acceptance by most authors that the Asclepiadaceae is nested within the Apocynaceae and can no longer be maintained as a separate family. These molecular studies, followed on from morphological studies which had already begun to question the distinction between the two families (Wanntorp 1989; Judd et al. 1994). In recent years much of the taxonomic literature on genera traditionally placed in the Asclepiadaceae has been published under the title of a work in the Apocynaceae, qualified by a reference to one of the three former Asclepiadaceae subfamilies (e.g. Livshultz et al. 2005). It may even be the case that the former Asclepiadaceae is not monophyletic and that two separate clades are independently nested within the Apocynaceae s.s. (Livshultz et al., in press). The Apocynaceae s.s. is very clearly paraphyletic. Whether one agrees with the notion that all families must be monophyletic or not there is little doubt, even from a more traditional perspective, that the characters used to delimit the Apocynaceae and Asclepiadaceae, particularly in the nature of the pollination system, are frequently not nearly as clearly demarcated as is often supposed (Goyder 1999). Resistance to change from authors on Apocynaceae s.s. and Asclepiadaceae has been largely absent although this does little to alter the fact that researchers continue to work largely in either one or the other part of the family making comprehensive floristic accounts to include all species of the Apocynaceae s.l. a near impossibility at this stage. Hence this flora account is really the publication of the Apocynaceae in the traditional sense, whilst recognising that it is merely an account of two of the subfamilies of the Apocynaceae s.l. This may not, however, impress strict adherents of monophyletic taxa because it is also clear that subfamilies Rauvolfioideae and Apocynaceae are paraphyletic (see below). What cannot be disputed though is how little is currently known about the taxonomy of most of the genera placed in the subfamilies traditionally included in the Asclepiadaceae. It will take many more years before accounts of these subfamilies will be ready for publication in Flora Malesiana and to wait to publish subfamilies Rauvolfioideae and Apocynoideae would be a disservice to Malesian botany.
Endress & Bruyns (2000) have published a classification of the combined family which includes five subfamilies. Two of these, Rauvolfioideae and Apocynoideae, constitute the Apocynaceae in the traditional sense and the other three, Periplocoideae, Secamonoideae and Asclepiadoideae, constitute the former Asclepiadaceae. They also included a tribal classification within these subfamilies, some of which are in need of substantial revision (Livshultz et al. in press; Simões et al. in press), particularly in subfamily Apocynoideae.
The Apocynaceae is a family in flux due to recent advances made in our understanding of the relationships between and within the families of the Gentianales through molecular phylogenetic studies (Sennblad & Bremer 1996, 2000, 2002; Endress et al. 1996; Sennblad et al. 1998; Potgieter & Albert 2001; Livshultz et al. in press; Simões et al. in press). The principal effect of this is the acceptance by most authors that the Asclepiadaceae is nested within the Apocynaceae and can no longer be maintained as a separate family. These molecular studies, followed on from morphological studies which had already begun to question the distinction between the two families (Wanntorp 1989; Judd et al. 1994). In recent years much of the taxonomic literature on genera traditionally placed in the Asclepiadaceae has been published under the title of a work in the Apocynaceae, qualified by a reference to one of the three former Asclepiadaceae subfamilies (e.g. Livshultz et al. 2005). It may even be the case that the former Asclepiadaceae is not monophyletic and that two separate clades are independently nested within the Apocynaceae s.s. (Livshultz et al., in press). The Apocynaceae s.s. is very clearly paraphyletic. Whether one agrees with the notion that all families must be monophyletic or not there is little doubt, even from a more traditional perspective, that the characters used to delimit the Apocynaceae and Asclepiadaceae, particularly in the nature of the pollination system, are frequently not nearly as clearly demarcated as is often supposed (Goyder 1999). Resistance to change from authors on Apocynaceae s.s. and Asclepiadaceae has been largely absent although this does little to alter the fact that researchers continue to work largely in either one or the other part of the family making comprehensive floristic accounts to include all species of the Apocynaceae s.l. a near impossibility at this stage. Hence this flora account is really the publication of the Apocynaceae in the traditional sense, whilst recognising that it is merely an account of two of the subfamilies of the Apocynaceae s.l. This may not, however, impress strict adherents of monophyletic taxa because it is also clear that subfamilies Rauvolfioideae and Apocynaceae are paraphyletic (see below). What cannot be disputed though is how little is currently known about the taxonomy of most of the genera placed in the subfamilies traditionally included in the Asclepiadaceae. It will take many more years before accounts of these subfamilies will be ready for publication in Flora Malesiana and to wait to publish subfamilies Rauvolfioideae and Apocynoideae would be a disservice to Malesian botany.
Endress & Bruyns (2000) have published a classification of the combined family which includes five subfamilies. Two of these, Rauvolfioideae and Apocynoideae, constitute the Apocynaceae in the traditional sense and the other three, Periplocoideae, Secamonoideae and Asclepiadoideae, constitute the former Asclepiadaceae. They also included a tribal classification within these subfamilies, some of which are in need of substantial revision (Livshultz et al. in press; Simões et al. in press), particularly in subfamily Apocynoideae.
Cytology
The most comprehensive information on the chromosomes of the Apocynaceae is by Van der Laan & Arends (1985) and, for the former Asclepiadaceae, Albers & Meve (2001). In subfamilies Rauvolfioideae and Apocynoideae basic chromosome numbers of x = 6, 8, 9, 10, 11, 12, 16, 18, 20, 21 and 23 have been found. The most common basic chromosome number is x = 11. Polyploidy has been reported within species (e.g. Rauvolfia serpentina), although these represent a small minority of species studied. Differing basic chromosome numbers within genera and/or infrageneric polyploidy has been reported for Alstonia (2n = 22, 42, 44, 88), Alyxia (2n = 36, 160-190), Carissa (2n = 22, 66), Kopsia (2n = 36, 72), Rauvolfia (2n = 22, 44, 66, 88) and Tabernaemontana (2n = 22, 66).
Uses
Information on uses for Apocynaceae species in Malesia has been collated in the PROSEA series from where the following information has been gleaned (see ). Almost all genera of Apocynaceae have species that are used to varying degrees and in a variety of ways. The principal uses of Apocynaceae species are, or were, in horticulture, medicine and the use of the latex. Some of the tree species have relatively minor uses in the timber industry and a few have edible fruits or leaves used for dyes.
Several Apocynaceae species are very familiar all through Southeast Asia from their frequent appearance in gardens and public spaces. The most commonly cultivated species are Allamanda catharctica, Allamanda schottii, Plumeria obtusa, Plumeria rubra, Thevetia peruviana, all from Central and South America, Nerium oleander from the Mediterranean region, Adenium obesum from Africa and Southwest Asia and Catharanthus roseus from Madagascar. Some native genera have commonly cultivated non-native species such as Tabernaemontana divaricata from Continental Asia, Carissa carandas from India and Kopsia fruticosa, probably native only in Burma. It is unclear whether the attractive shrub Wrightia religiosa is native in Peninsular Malaysia or only further north, it is so commonly grown that its natural distribution has become rather obscure. Relatively few native species are as popular in gardens although a number of Tabernaemontana species are grown and Cerbera odollam is frequently grown as a street tree.
There is an increasingly large body of literature on the medicinal uses of Apocynaceae species. Some of this information is summarised under the relevant species below. The most promising medicinal plant in the family to date is Catharanthus roseus from Madagascar but now widely cultivated and naturalised throughout the tropics. In traditional medicine a decoction of all parts of the plant is used to treat malaria, diarrhoea, diabetes, cancer and skin diseases. Extracts prepared from the leaves are used externally as an antiseptic on wounds, against haemorrhage and as a treatment for toothache. It is also said to be effective against indigestion, dysentery and wasp stings and can be used as vomitive, purgative, vermifuge and a general purifier of the body. Indeed this list of traditional uses has led to pharmaceutical investigation and extracts of the plant are now industrially produced and prescribed in a wide range of anti-cancer chemotherapies. Extracts of the roots are also used to treat some of the effects of senility, dizziness, tinnitus, cranial traumas and other neurological problems by increasing the blood flow to the brain. Some antiviral, fungicidal and nematocidal activity has also been reported for this species, as well as its use in the protection of stored grain against insect attack. However, in all these treatments the toxic effects of the plant must also be considered. Very little of the commercial cultivation of Catharanthus roseus for the medical industry occurs in Southeast Asia.
The only other species to have attracted a similar level of attention are in the genus Rauvolfia, particularly R. serpentina. It has been used in the ancient practice of Ayurvedic medicine for the treatment of snake bites, mental illnesses and epilepsy. In other areas it has been used as a treatment for high blood pressure, for a wide range of mental health problems, liver diseases, dysentery and in childbirth. It has also been used externally for eye problems and as a treatment for wounds. Its commercial exploitation, however, has been much reduced in recent years because of the harmful side effects of the most important of the alkaloids that were extracted for the medical industry, reserpine. Other extracts are still used for the treatment of psychological and behavioural problems. Uses more specific to individual species are described under those species below. Several other species have important medicinal uses but are described under the relevant taxa below.
Some species were previously important for the production of rubber before Hevea brasiliensis came to dominate the industry. In particular the two Dyera species were tapped for making rubber and then later for the manufacture of chewing gum. However, this latter use is also now in decline. The latex of other species such as Chonemorpha verrucosa, Leuconotis eugeniifolius, Leuconotis griffithii, Melodinus orientalis, Micrechites serpyllifolius, Urceola brachysepala, Urceola elastica, Urceola lucida, Urceola micrantha, Urceola torulosa, Willughbeia angustifolia, Willughbeia coriacea, Willughbeia edulis, Willughbeia flavescens, Willughbeia grandiflora, Willughbeia oblonga and Willughbeia tenuiflora have also been exploited for use as rubber of varying quality.
A few species have edible fruits, particularly from subfamily Rauvolfioideae where berries occur. Ochrosia ackeringae is said to have edible seeds.
The trees in the Apocynaceae are generally not particularly important in the timber trade except possibly for some species of Alstonia which are separable into a light hardwood, corresponding to section Alstonia, and a medium-heavy hardwood, corresponding to section Monuraspermum. The latter can be used in construction. Further information can be found under the species. Most of the other tree species in the Apocynaceae yield a wood which is not of great quality and are used for small items like axe handles, light furniture, carving etc.
Several Apocynaceae species are very familiar all through Southeast Asia from their frequent appearance in gardens and public spaces. The most commonly cultivated species are Allamanda catharctica, Allamanda schottii, Plumeria obtusa, Plumeria rubra, Thevetia peruviana, all from Central and South America, Nerium oleander from the Mediterranean region, Adenium obesum from Africa and Southwest Asia and Catharanthus roseus from Madagascar. Some native genera have commonly cultivated non-native species such as Tabernaemontana divaricata from Continental Asia, Carissa carandas from India and Kopsia fruticosa, probably native only in Burma. It is unclear whether the attractive shrub Wrightia religiosa is native in Peninsular Malaysia or only further north, it is so commonly grown that its natural distribution has become rather obscure. Relatively few native species are as popular in gardens although a number of Tabernaemontana species are grown and Cerbera odollam is frequently grown as a street tree.
There is an increasingly large body of literature on the medicinal uses of Apocynaceae species. Some of this information is summarised under the relevant species below. The most promising medicinal plant in the family to date is Catharanthus roseus from Madagascar but now widely cultivated and naturalised throughout the tropics. In traditional medicine a decoction of all parts of the plant is used to treat malaria, diarrhoea, diabetes, cancer and skin diseases. Extracts prepared from the leaves are used externally as an antiseptic on wounds, against haemorrhage and as a treatment for toothache. It is also said to be effective against indigestion, dysentery and wasp stings and can be used as vomitive, purgative, vermifuge and a general purifier of the body. Indeed this list of traditional uses has led to pharmaceutical investigation and extracts of the plant are now industrially produced and prescribed in a wide range of anti-cancer chemotherapies. Extracts of the roots are also used to treat some of the effects of senility, dizziness, tinnitus, cranial traumas and other neurological problems by increasing the blood flow to the brain. Some antiviral, fungicidal and nematocidal activity has also been reported for this species, as well as its use in the protection of stored grain against insect attack. However, in all these treatments the toxic effects of the plant must also be considered. Very little of the commercial cultivation of Catharanthus roseus for the medical industry occurs in Southeast Asia.
The only other species to have attracted a similar level of attention are in the genus Rauvolfia, particularly R. serpentina. It has been used in the ancient practice of Ayurvedic medicine for the treatment of snake bites, mental illnesses and epilepsy. In other areas it has been used as a treatment for high blood pressure, for a wide range of mental health problems, liver diseases, dysentery and in childbirth. It has also been used externally for eye problems and as a treatment for wounds. Its commercial exploitation, however, has been much reduced in recent years because of the harmful side effects of the most important of the alkaloids that were extracted for the medical industry, reserpine. Other extracts are still used for the treatment of psychological and behavioural problems. Uses more specific to individual species are described under those species below. Several other species have important medicinal uses but are described under the relevant taxa below.
Some species were previously important for the production of rubber before Hevea brasiliensis came to dominate the industry. In particular the two Dyera species were tapped for making rubber and then later for the manufacture of chewing gum. However, this latter use is also now in decline. The latex of other species such as Chonemorpha verrucosa, Leuconotis eugeniifolius, Leuconotis griffithii, Melodinus orientalis, Micrechites serpyllifolius, Urceola brachysepala, Urceola elastica, Urceola lucida, Urceola micrantha, Urceola torulosa, Willughbeia angustifolia, Willughbeia coriacea, Willughbeia edulis, Willughbeia flavescens, Willughbeia grandiflora, Willughbeia oblonga and Willughbeia tenuiflora have also been exploited for use as rubber of varying quality.
A few species have edible fruits, particularly from subfamily Rauvolfioideae where berries occur. Ochrosia ackeringae is said to have edible seeds.
The trees in the Apocynaceae are generally not particularly important in the timber trade except possibly for some species of Alstonia which are separable into a light hardwood, corresponding to section Alstonia, and a medium-heavy hardwood, corresponding to section Monuraspermum. The latter can be used in construction. Further information can be found under the species. Most of the other tree species in the Apocynaceae yield a wood which is not of great quality and are used for small items like axe handles, light furniture, carving etc.
Notes
Very many species in the Apocynaceae were originally described in the genus Echites which was later split up to form many of the genera of subfamily Apocynoideae. A number of these genera were given a suffix of -echites, e.g. Micrechites, Papuechites, Sindechites. All of these have always been treated as feminine. However, following Art. 62.4 of the Code all of these names, unfortunately, have to be treated as masculine. Therefore, these names are herein treated as masculine and without the reader being further informed that the name was originally published in the feminine.
Citation
Smythies 1965: Common Sarawak Trees. p 17
G. Don 1837 – In: Gen. Hist. p 69
Markgr. 1926 – In: Nova Guinea. p 278
Ridl. 1923 – In: Fl. Malay Penins. p 320
Kochummen 1997: Tree Fl. Pasoh Forest. p 150
Benth. & Hook.f. 1876 – In: Gen. Pl. p 681
H. Huber 1973 – In: Abeyw. (ed.), Revis. Handb. Fl. Ceylon 1. p 1
A.DC. 1844 – In: Prodr. p 317
D.J. Middleton 1999 – In: Fl. Thailand. p 1
K. Schum. 1895 – In: Engl. & Prantl., Nat. Pflanzenfam. 4. p 109
F.G. Browne 1955: Forest Trees Sarawak & Brunei. p 60
Backer & Bakh.f. 1965 – In: Fl. Java. p 218
Kessler et al. 2002 – In: Blumea. p 13
Cockburn 1976 – In: Trees Sabah. p 13
M. Gangop. & Chakr. 1992 – In: J. Econ. Taxon. Bot. p 27
T.C. Huang 1986 – In: Taiwania. p 89
Corner 1952 – In: Wayside Trees Malaya, ed. 2. p 137
Utteridge 2006 – In: R.J. Johns et al., Alp. Subalp. Fl. Mount Jaya. p 187
P.F. Burgess 1966: Timbers Sabah. p 39
King & Gamble 1907 – In: J. Asiat. Soc. Bengal. p 387
Kessler & Sidiy. 1994: Trees Balikpapan-Samarinda. p 50
D.J. Middleton 2004 – In: Tree Fl. Sabah & Sarawak. p 1
P.T. Li et al. 1995 – In: Fl. China. p 143
H. Huber 1983 – In: Abeyw. (ed.), Revis. Handb. Fl. Ceylon 4. p 25
P.S. Ashton 1988 – In: Man. non-Dipt. Trees Sarawak. p 16
Adans. 1927 – In: Bot. Jahrb. Syst. p 164